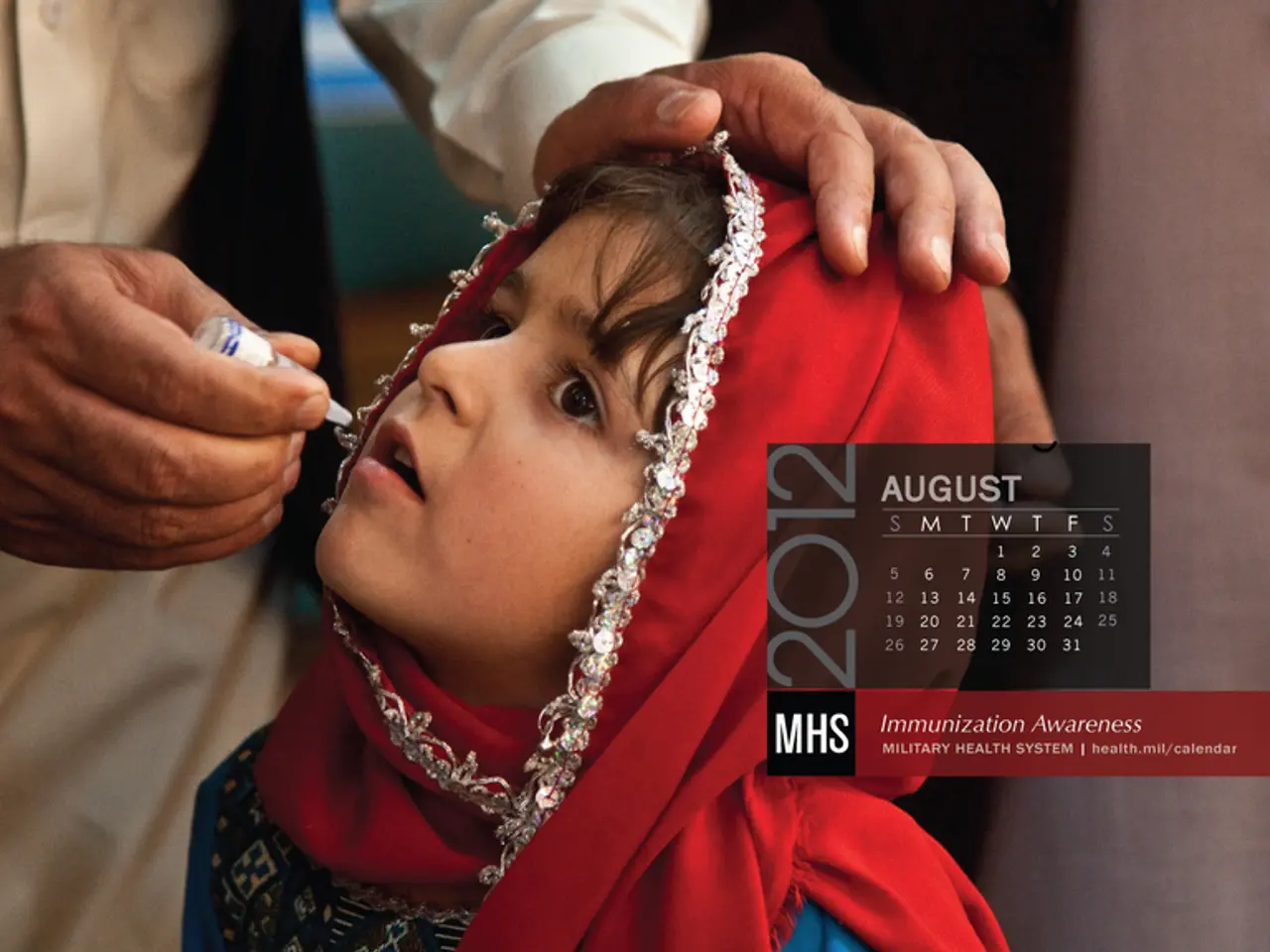
Approval granted by FDA for over-the-counter nasal flu vaccine for individuals aged 2-49 years old at home
The Food and Drug Administration (FDA) has approved a nasal spray flu vaccine, FluMist, for self-administration at home by consumers aged 2 to 49. This marks a significant step forward in public health, as FluMist Home allows eligible adults to self-administer and caregivers to administer to children intranasally, offering greater convenience, flexibility, and accessibility.
Manufactured by AstraZeneca, FluMist works by stimulating the immune system in the lining of the nose and throat to build immunity to the virus. The viruses in FluMist are weakened strains of the live influenza virus, and the vaccine does not spark infection.
To ensure safety and eligibility, consumers must complete an eligibility assessment on the pharmacy's website before receiving FluMist. Key eligibility requirements include adults aged 18 to 49 years being able to self-administer the nasal spray vaccine, while children aged 2 to 17 years can receive the vaccine administered by a parent or caregiver.
The vaccine is currently available for delivery and self-administration only in 34 states due to state pharmacy regulations, including California, Florida, New York, Texas, and Washington.
To use FluMist at home, order the vaccine online, complete a brief medical screening reviewed by a healthcare provider partnered with AstraZeneca to confirm eligibility. The vaccine is shipped refrigerated with a special tag ensuring proper temperature during transit. Upon receipt, keep the vaccine refrigerated until use. The vaccine is delivered intranasally: spray once into each nostril. Users or caregivers should administer the spray by squatting or sitting and gently inserting the nozzle to spray.
It is normal to experience a slight tickle, dripping, or sneezing during administration. Individuals with a head cold or thick mucus should wait a couple of days before administration, as nasal congestion can reduce vaccine effectiveness. After vaccination, it typically takes about 2 weeks to develop flu protection. Yearly vaccination is recommended for best protection.
Most health insurance plans cover the vaccine cost, though shipping and handling fee is approximately $8.99. The convenience and needle-free delivery aim to boost vaccination rates, especially as the previous flu season was notably severe.
The FDA's approval of FluMist demonstrates its commitment to advancing public health, and Peter Marks, M.D., Ph.D., director of the FDA's Center for Biologics Evaluation and Research, stated that the approval adds another option for vaccination against influenza disease. Getting vaccinated each year is the best way to prevent influenza, according to the FDA, and FluMist Home offers a new, accessible method for achieving this goal.
References:
- FluMist Home
- AstraZeneca's FluMist Home
- FDA approves FluMist for at-home use
- FluMist Home Instructions
- FluMist Home Cost
This decision by the Food and Drug Administration (FDA) to approve FluMist Home, a nasal spray flu vaccine for self-administration at home in the health-and-wellness sector, is a significant stride in promoting lifestyle changes for greater vaccine accessibility. Manufactured by AstraZeneca, the vaccine targets consumers aged 2 to 49, stimulating the immune system in the lining of the nose and throat, as part of home-and-garden measures to combat the virus.